1.5.1 Distinguish between the terms solute, solvent, solution and concentration (g dm^-3 and mol dm^-3)
Solutions are a mixture between two components, the less abundant component is the solute and the more abundant is the solvent.
Solute can be solid, liquid or gas.
Solvent is generally a liquid.
Salt water is a solution with salt as the solute and water as the solvent.
They are called aqueous solutions and are given the state symbol (aq).
Concentration has two units, either g dm^-3 or mol dm^-3
Concentration = Mass of solute / Volume of solvent
Concentration = Moles of solute / Volume of solvent
g dm^-3 = mol dm^-3 x molar mass
1.5.2 Solve problems involving concentration, amount of solute and volume of solute.
This equation will be least likely to be a question on its own. However it is used in a lot of different questions to test your basic knowledge.
Examiner's Tip : In Paper 1 you can find the best solution with less precise relative atomic mass values. This makes the calculation easier and saves time. For papers 2 and 3 estimate the answer before you use a calculator. This will help spot careless mistakes.
Sunday, 15 September 2013
Topic 1.4: Mass and gaseous volume relationships in chemical reactions
1.4.1 Calculate theoretical yields from chemical equations
Given the mass of the reactants, using mole ratio determine the mass of the products.
Divide Mass by Molar Mass of reactant.
Use Mole Ratio to convert the moles of reactant to moles of product
Multiple Moles of Products to Molar Mass of Product
Receive final answer for Theoretical Yield for mass of Product.
1.4.2 Determine the limiting reactant and the reactant in excess when quantities of reacting substances are given.
If one mole of substances reacts with one mole of substances, unless you have exact mass of each substances there will always be a limiting reactant and reactant in excess.
For example, if there are more hydrogen than oxygen required in one chemical reaction, then oxygen is the limiting reactant while hydrogen is in excess. Hence, "limiting" the reaction and "excess" which is more than enough.
Divide both reactant's mass by their respective molar mass to receive the moles.
The reactant with less moles is the limiting reagent.
The reactant with more moles is the reactant in excess.
1.4.3 Solve problems involving theoretical, experimental and percentage yield
Chemical reactions aren't completely efficient. Therefore the experimental yield (actual yield), is generally less than the theoretical yield predicted.
Percentage yield = Experimental yield / Theoretical yield x 100%
Change the equation around to receive different forms of the same equation.
1.4.4 Apply Avogadro's law to calculate reacting volumes of gases.
Avogadro's law states that equal volume of different gases contain equal numbers of particles at the same temperature and pressure.
1.4.5 Apply the concept of molar volume at standard temperature and pressure in calculations.
All gases have the same molar volume at the same temperature and pressure. The standard conditions of temperature and pressure (STP) are 273 K (0 degree Celcius) and 100 kPa pressure.
One mole of gas occupies 22.4 dm^3 under STP while it occupies 24.0 dm^3 under RTP (room temperature which is 298 K)
Number of moles = Volume / Molar Volume (22.4 dm^3 at STP and 24.0 dm^3 at RTP)
1.4.6 Solve problems involving the relationship between temperature, pressure and volume for a fixed mass of an ideal gas.
There are three laws of Gas.
Boyle's Law state that pressure of a gas is inversely proportional to the volume.
Gay-Lussac's Law state that pressure of a gas is directly proportional to the temperature
Charles's Law state that the volume is directly proportional to the temperature.
The Combined Gas Law simply combines them all to one equation.
Note: Temperature is measured in Kelvins.
How to use the Combined Gas Law, simply input the numbers into the equation. If the question states that one of them is constant, then you can remove it to use one of the three simple laws instead.
1.4.7 Solve problems using the ideal gas equation, PV = nRT
This is the Combined Gas Law
The Ideal Gas Equation adds the Avogadro's Law to the Combined Gas Law
P = Pressure
V = Volume
n = number of moles
T = Temperature in Kelvins
R = Gas Constant
The Gas Constant is 8.31 J K^-1 mol^-1.
The SI unit is Joules / (Kelvin x Moles)
1.4.8 Analysis graphs relating to the ideal gas equation.
Boyle's Law Graph
Gay-Lussac's Law Graph
Charles's Law Graph
Given the mass of the reactants, using mole ratio determine the mass of the products.
Divide Mass by Molar Mass of reactant.
Use Mole Ratio to convert the moles of reactant to moles of product
Multiple Moles of Products to Molar Mass of Product
Receive final answer for Theoretical Yield for mass of Product.
1.4.2 Determine the limiting reactant and the reactant in excess when quantities of reacting substances are given.
If one mole of substances reacts with one mole of substances, unless you have exact mass of each substances there will always be a limiting reactant and reactant in excess.
For example, if there are more hydrogen than oxygen required in one chemical reaction, then oxygen is the limiting reactant while hydrogen is in excess. Hence, "limiting" the reaction and "excess" which is more than enough.
Divide both reactant's mass by their respective molar mass to receive the moles.
The reactant with less moles is the limiting reagent.
The reactant with more moles is the reactant in excess.
1.4.3 Solve problems involving theoretical, experimental and percentage yield
Chemical reactions aren't completely efficient. Therefore the experimental yield (actual yield), is generally less than the theoretical yield predicted.
Percentage yield = Experimental yield / Theoretical yield x 100%
Change the equation around to receive different forms of the same equation.
1.4.4 Apply Avogadro's law to calculate reacting volumes of gases.
Avogadro's law states that equal volume of different gases contain equal numbers of particles at the same temperature and pressure.
1.4.5 Apply the concept of molar volume at standard temperature and pressure in calculations.
All gases have the same molar volume at the same temperature and pressure. The standard conditions of temperature and pressure (STP) are 273 K (0 degree Celcius) and 100 kPa pressure.
One mole of gas occupies 22.4 dm^3 under STP while it occupies 24.0 dm^3 under RTP (room temperature which is 298 K)
Number of moles = Volume / Molar Volume (22.4 dm^3 at STP and 24.0 dm^3 at RTP)
1.4.6 Solve problems involving the relationship between temperature, pressure and volume for a fixed mass of an ideal gas.
There are three laws of Gas.
Boyle's Law state that pressure of a gas is inversely proportional to the volume.
Gay-Lussac's Law state that pressure of a gas is directly proportional to the temperature
Charles's Law state that the volume is directly proportional to the temperature.
The Combined Gas Law simply combines them all to one equation.
Note: Temperature is measured in Kelvins.
How to use the Combined Gas Law, simply input the numbers into the equation. If the question states that one of them is constant, then you can remove it to use one of the three simple laws instead.
1.4.7 Solve problems using the ideal gas equation, PV = nRT
This is the Combined Gas Law
The Ideal Gas Equation adds the Avogadro's Law to the Combined Gas Law
P = Pressure
V = Volume
n = number of moles
T = Temperature in Kelvins
R = Gas Constant
The Gas Constant is 8.31 J K^-1 mol^-1.
The SI unit is Joules / (Kelvin x Moles)
1.4.8 Analysis graphs relating to the ideal gas equation.
Boyle's Law Graph
Charles's Law Graph
Topic 1.3: Chemical Equations
1.3.1 Deduce chemical equations when all reactants and products are given.
A chemical equation provides a balance sheet which allows us to monitor these changes as reactants are transformed into products.
All reactants are on the left side of the equation, while all products are on the right side of the equation
1.3.2 Identify the mole ratio of any two species in a chemical equation.
The number of atoms of each element must be the same on both sides of the equation.
Simply saying, 1 mole of methane reacts with 2 moles of oxygen to create 1 mole of carbon dioxide and 2 moles of water.
The Mole Ratio is used to balance out the equation. Be sure that the number of atoms of each element must be the same on both sides of the equation.
1.3.3 Apply the state symbols (s), (l), (g) and (aq)
State symbols simply state the state of matter the element is in during the chemical reaction.
A chemical equation provides a balance sheet which allows us to monitor these changes as reactants are transformed into products.
All reactants are on the left side of the equation, while all products are on the right side of the equation
1.3.2 Identify the mole ratio of any two species in a chemical equation.
The number of atoms of each element must be the same on both sides of the equation.
Simply saying, 1 mole of methane reacts with 2 moles of oxygen to create 1 mole of carbon dioxide and 2 moles of water.
The Mole Ratio is used to balance out the equation. Be sure that the number of atoms of each element must be the same on both sides of the equation.
1.3.3 Apply the state symbols (s), (l), (g) and (aq)
State symbols simply state the state of matter the element is in during the chemical reaction.
Topic 1.2: Formulas
1.2.1 Define the terms relative atomic mass (Ar) and relative molecular mass (Mr)
Relative Atomic Mass: The mass of one mole of atoms of an element is Relative Atomic Mass (Ar), expressed in grams.
But masses of all elements are in the range of 10^-24 to 10^-22 grams and these numbers are way too inconvenient to use practically. The mass was then recorded relative to the common element carbon. Carbon-12 is the most abundant isotope of carbon, but because Carbon-13 and Carbon-14 also exist, the average relative atomic mass (Ar) is greater than 12.
Relative Molecular Mass: The Relative Molecular Mass (Mr) is calculated by adding the relative atoic masses of the atoms making up the molecule.
1.2.2 Calculate the mass of one mole of a species from its formula
Mass = Molar Mass x Moles
1.2.3 Solve problems involving the relationship between the amount of substances in moles, mass and molar mass.
Simple tips, remember the formula is interchangeable
Mass = Molar Mass x Moles
Molar Mass = Mass / Moles
Moles = Mass / Molar Mass
Also Moles is constant in an chemical equation, except when there is a Mole Ratio.
1.2.4 Distinguish between the terms empirical formula and molecular formula.
Empirical Formula is the simplest ratio known to a chemical formula
Molecular Formula is a multiple of the empirical formula, can only be determined once the relative molecular mass is known. It also show the actual number of atoms in the molecule
1.2.5 Determine the empirical formula from the percentage composition or from other experimental data.
Determine the elements in the molecule.
Fine the Molar Mass of these individual elements.
Divide the percentage composition of each element to their respective Molar Mass to determine moles
Find the ratio between the moles to create the Empirical Formula
1.2.6 Determine the molecular formula when given both the empirical formula and the experimental data.
Experimental data should include the Molar Mass of the Molecular Formula.
Find Molar Mass for Empirical Formula
Divide the Molar Mass of Molecular Formula by the Molar Mass for Empirical Formula.
Multiple all elements by the same number produced to receive the Molecular Formula.
Relative Atomic Mass: The mass of one mole of atoms of an element is Relative Atomic Mass (Ar), expressed in grams.
But masses of all elements are in the range of 10^-24 to 10^-22 grams and these numbers are way too inconvenient to use practically. The mass was then recorded relative to the common element carbon. Carbon-12 is the most abundant isotope of carbon, but because Carbon-13 and Carbon-14 also exist, the average relative atomic mass (Ar) is greater than 12.
Relative Molecular Mass: The Relative Molecular Mass (Mr) is calculated by adding the relative atoic masses of the atoms making up the molecule.
1.2.2 Calculate the mass of one mole of a species from its formula
Mass = Molar Mass x Moles
1.2.3 Solve problems involving the relationship between the amount of substances in moles, mass and molar mass.
Simple tips, remember the formula is interchangeable
Mass = Molar Mass x Moles
Molar Mass = Mass / Moles
Moles = Mass / Molar Mass
Also Moles is constant in an chemical equation, except when there is a Mole Ratio.
1.2.4 Distinguish between the terms empirical formula and molecular formula.
Empirical Formula is the simplest ratio known to a chemical formula
Molecular Formula is a multiple of the empirical formula, can only be determined once the relative molecular mass is known. It also show the actual number of atoms in the molecule
1.2.5 Determine the empirical formula from the percentage composition or from other experimental data.
Determine the elements in the molecule.
Fine the Molar Mass of these individual elements.
Divide the percentage composition of each element to their respective Molar Mass to determine moles
Find the ratio between the moles to create the Empirical Formula
1.2.6 Determine the molecular formula when given both the empirical formula and the experimental data.
Experimental data should include the Molar Mass of the Molecular Formula.
Find Molar Mass for Empirical Formula
Divide the Molar Mass of Molecular Formula by the Molar Mass for Empirical Formula.
Multiple all elements by the same number produced to receive the Molecular Formula.
Topic 1.1: The mole concept and Avogadro's constant
1.1.1 Apply the mole concept to substances.
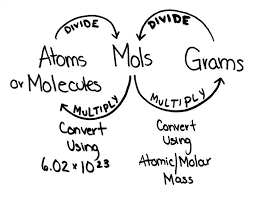
The mole concept applies to all kinds of particles: atoms, molecules, ions, electrons, formula units, and so on. The amount of substance is measured in moles (mol). The approximate Avogadro's constant (L), 6.02 x 10^23 mol^-1, should be known.
Easy way to remember, "1 mole x 1 molar mass = 1 g of mass"
"1 mole" is how many atom or molecule
"1 molar mass" is size of atom or molecule
"1 gram of mass" is mass in total.
Also, 1 mole = Avoagadro's constant.
1.1.2 Determine the number of particles and the amount of substance (in moles).
Convert between the number of particles and amount of substances.
Easy Conversions
1 mol = 6.02 x 10^23 particles
2 mol = 2 x 6.02 x 10 ^23 particles
The mole concept applies to all kinds of particles: atoms, molecules, ions, electrons, formula units, and so on. The amount of substance is measured in moles (mol). The approximate Avogadro's constant (L), 6.02 x 10^23 mol^-1, should be known.
Easy way to remember, "1 mole x 1 molar mass = 1 g of mass"
"1 mole" is how many atom or molecule
"1 molar mass" is size of atom or molecule
"1 gram of mass" is mass in total.
Also, 1 mole = Avoagadro's constant.
1.1.2 Determine the number of particles and the amount of substance (in moles).
Convert between the number of particles and amount of substances.
Easy Conversions
1 mol = 6.02 x 10^23 particles
2 mol = 2 x 6.02 x 10 ^23 particles
Sunday, 8 September 2013
Topic 2.1: The Atom
2.1.1 State the position of protons, neutrons and electrons in the atom.
Proton and neutrons are both present in the nucleus of the atom, they are also known as nucleons. While electrons are present in the energy levels.
2.1.2 State the relative masses of relative charges of protons, neutrons and electrons.
Protons have relative mass of 1 and relative charge of +1. It has a positive charge.
Neutrons have relative mass of 1 and relative charge of 0. It has no charge.
Electron have relative mass of 5 x 10^4 or 0.0005 and relative charge of -1. It has a negative charge.
2.1.3 Define the terms mass number (A), atomic number (Z), and isotopes of an element.
Mass number or relative atomic mass is how heavy an atom is compared to hydrogen. a.k.a (A)
Atomic number is the number of proton in an atom. a.k.a (Z)
Isotopes are the same element with the same number of proton but different number of neutrons.
Protium, Deuterium and Tritium are all isotopes of hydrogen. (Example)
2.1.4 Deduce the symbol for an isotope given its mass number and atomic number.
The symbol of an element is found by matching the "atomic number" with its corresponding one on the periodic table. It is important to use the atomic number because there are individual unique element for each atomic number.
2.1.5 Calculate the number of protons, neutrons and electrons in atoms and ions from mass number, atomic number and charge.
To calculate the number of protons, simply find Z or look for the chemical symbol in the periodic table.
To calculate the number of neutrons, simply find A-Z. Since A is the number of both proton and neutrons, we can get the only neutron by subtracting the protons.
To calculate the number of electrons, simply find Z and its charge (n). Since protons should equal electrons in an atom, then the electron will be Z. However, if it is an ion then extra steps are required. If the charge is +1, then subtract one from Z and vice versa.
2.1.6 Compare the properties of the isotopes of an element.
Isotopes show the same chemical properties, as a difference in the number of neutrons makes no difference to how they react and so they occupy the same place in the Periodic Table.
The difference in mass lead to different physical properties such as boiling and melting points. Heavier isotopes move more slowly at a given temperature and these differences can be used to separate isotopes
2.1.7 Discuss the uses of radioisotopes.
Carbon 14 dating
Carbon 14 has eight neutrons which is too many to be stable. It can reduce the neutron to proton ratio when a neutron changes to a proton. This is beta radiation.
The relative abundance of carbon-14 present in living organism is constant due to breathing and carbon dioxide. However, when an organisms dies, it no longer absorbs carbon 14 and carbon 14 fall owing to nuclear decay. As this process occurs at a regular rate, we can determine the death of an organism to 5730 years.
Cobalt-60 Radiotherapy
Treatment of cancer and other diseases using ionizing radiation. The treatment damages the genetic material inside a cell and making it impossible to grow, though this damages both cancer cells and normal cells.
Iodine 131 Medical Tracer
Iodine 131 is an emitter of both beta and gamma rays can be used to investigate the activity of the thyroid gland and treat thyroid cancer It has a half-life of 8 days so it is quickly eliminated from the body.
Proton and neutrons are both present in the nucleus of the atom, they are also known as nucleons. While electrons are present in the energy levels.
2.1.2 State the relative masses of relative charges of protons, neutrons and electrons.
Protons have relative mass of 1 and relative charge of +1. It has a positive charge.
Neutrons have relative mass of 1 and relative charge of 0. It has no charge.
Electron have relative mass of 5 x 10^4 or 0.0005 and relative charge of -1. It has a negative charge.
2.1.3 Define the terms mass number (A), atomic number (Z), and isotopes of an element.
Mass number or relative atomic mass is how heavy an atom is compared to hydrogen. a.k.a (A)
Atomic number is the number of proton in an atom. a.k.a (Z)
Isotopes are the same element with the same number of proton but different number of neutrons.
Protium, Deuterium and Tritium are all isotopes of hydrogen. (Example)
2.1.4 Deduce the symbol for an isotope given its mass number and atomic number.
The symbol of an element is found by matching the "atomic number" with its corresponding one on the periodic table. It is important to use the atomic number because there are individual unique element for each atomic number.
2.1.5 Calculate the number of protons, neutrons and electrons in atoms and ions from mass number, atomic number and charge.
To calculate the number of protons, simply find Z or look for the chemical symbol in the periodic table.
To calculate the number of neutrons, simply find A-Z. Since A is the number of both proton and neutrons, we can get the only neutron by subtracting the protons.
To calculate the number of electrons, simply find Z and its charge (n). Since protons should equal electrons in an atom, then the electron will be Z. However, if it is an ion then extra steps are required. If the charge is +1, then subtract one from Z and vice versa.
2.1.6 Compare the properties of the isotopes of an element.
Isotopes show the same chemical properties, as a difference in the number of neutrons makes no difference to how they react and so they occupy the same place in the Periodic Table.
The difference in mass lead to different physical properties such as boiling and melting points. Heavier isotopes move more slowly at a given temperature and these differences can be used to separate isotopes
2.1.7 Discuss the uses of radioisotopes.
Carbon 14 dating
Carbon 14 has eight neutrons which is too many to be stable. It can reduce the neutron to proton ratio when a neutron changes to a proton. This is beta radiation.
The relative abundance of carbon-14 present in living organism is constant due to breathing and carbon dioxide. However, when an organisms dies, it no longer absorbs carbon 14 and carbon 14 fall owing to nuclear decay. As this process occurs at a regular rate, we can determine the death of an organism to 5730 years.
Cobalt-60 Radiotherapy
Treatment of cancer and other diseases using ionizing radiation. The treatment damages the genetic material inside a cell and making it impossible to grow, though this damages both cancer cells and normal cells.
Iodine 131 Medical Tracer
Iodine 131 is an emitter of both beta and gamma rays can be used to investigate the activity of the thyroid gland and treat thyroid cancer It has a half-life of 8 days so it is quickly eliminated from the body.
Topic 2: The Atomic Structure
Topic 2 of the IB HL Chemistry syllabus is the Quantitative Chemistry. IBO recommends to spend 4 hours on this topic.
This topic has 3 sub-chapters: "The atom", "The mass spectrometer" and "Electron Arrangement". Each are separated with numerical values in order of mentioned.
These are all basic syllabus statements, it is recommended to bring a Casio Graphical Calculator instead of Texas. Casio Calculators have the periodic table installed already.
This topic has 3 sub-chapters: "The atom", "The mass spectrometer" and "Electron Arrangement". Each are separated with numerical values in order of mentioned.
These are all basic syllabus statements, it is recommended to bring a Casio Graphical Calculator instead of Texas. Casio Calculators have the periodic table installed already.
Subscribe to:
Comments (Atom)

