Homologous series is the name given to a group of compounds that can be described by a general formula
- All members of a homologous series have similar chemical properties
- The next member in a homologous series deiffers by a CH2 group (called a repeating unit)
- This causes a gradual change in physical properties as the chain length increases.
10.1.2 Predict and explain the trends in boiling points of members of a homologous series
Boiling point increases as chain length increases. This is due to an increase in Van De Waal forces between the molecules as the molar mass and surface area increases.
10.1.3 Distinguish between empirical, molecular and structural formulas
Organic structures can be represented in a variety of ways:
Empirical Formula is the simplest ratio of atoms of each elements in a compound
Molecular Formula is the actual number of atoms of each element presented in the compound
Full structural formula shows every bond and atom. Usually 90 and 180 degrees angles are used to show the bonds because this is the clearest representation on a 2-dimensional page,
Condensed structural formula often omits bonds where they can be assumed, and group atoms together. It contains the minimum information needed to describe the molecule non-ambiguously - in other words there is only one possible structure that could be described by this formula.
10.1.4 Describe structural isomers as compounds with the same molecular formula but with different arrangements of atoms
Structural isomers are different arrangements of the same atoms but make different molecules. Each isomer is a distinct compound, having unique physical and chemical properties.
10.1.5 Deduce structural formulas for the isomers of the non-cyclic alkanes up to (6 carbons) C6
Read the name of the isomer, and deduce the shape using the knowledge gained from 10.1.6
10.1.6 Apply IUPAC rules for naming the isomers of the non-cyclic alkanes up to (6 carbons) C6
Identify the longest carbon atom chain, it becomes the stem of the name.
1 - meth
2 - eth
3 - prop
4 - but
5 - pent
6 - hex
Side chains are placed in the suffix (-ane), are known as substituents. The number in front shows the number of the carbon it is linked to.
methyl, ethyl, propyl...
10.1.7 Deduce structural formulas for the isomers of the straight-chain alkenes up to (6 carbons) C6
Read the name of the isomer, and deduce the shape using the knowledge gained from 10.1.8
10.1.8 Apply IUPAC rules for naming the isomers of the straight-chain alkenes up to (6 carbons ) C6
Identify the longest carbon atom chain, it becomes the stem of the name.
1 - meth
2 - eth
3 - prop
4 - but
5 - pent
6 - hex
Side chains are placed in the suffix (-ene), are known as substituents. The number in front shows the number of the carbon it is linked to.
The number in the middle signifies where the double bond is. Trans and Cis show how the ethene is connected.
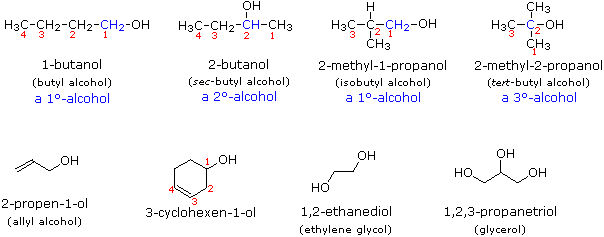
10.1.9 Deduce structural formulas for compounds containing up to six carbon atoms with one of the following functional groups: alcohol, aldehyde, ketone, carboxylic acid and halide
This will be discussed in 10.1.10
10.1.10 Apply IUPAC rules for naming compounds containing up to six carbon atoms with one of the following functional groups: alcohol, aldehyde, ketone, carboxylic acid and halide
Similar to previous syllabus statements, alcohol's suffix is (-anol), aldehyde's suffix is (-anal), ketone's suffix is (-anone), carboxylic acid's suffix is (-anoic acid). Halide's have a prefix dependent on the type of halogen. (Fluoro-, Chloro-, Bromo-, Iodo-)
Alcohol
Aldehyde
Ketone
Carboxylic acid
Halide
10.1.11 Identify the following functional group when present in structural formulas: amino (NH2), benzene ring and esters (RCOOR)
When looking at the structural formula double check for any amino, benzene ring and esters
10.1.12 Identify primary, secondary and tertiary carbon atoms in alcohols and halogenoalkanes
Identifying what degree carbon depends on how many other carbons it is connected to.
10.1.13 Discuss the volatility and solubility in water of compounds containing the functional groups listed in 10.1.9
Volatility is a measure of how easily a substance changes into the gaseous state - high volatility means that the compound has a low boiling point. Volatility is dependent on overcoming the forces between the molecules, so the stronger the intermolecular forces, the higher the boiling point.
Most volatile
Alkane
Halogenalkane
Aldehyde
Ketone
Alcohol
Carboxylic acid
Least volatile
Solubility is largely determined by the extent to which the solute molecules are able to interact and form hydrogen bonds with water.

No comments:
Post a Comment